Diagenesis: Stereoisomerism
Stereochemical changes:
|
During diagenesis the stereochemical configuration of certain sedimentary biomolecules changes to be in equilibrium with the conditions encountered in the sediments.
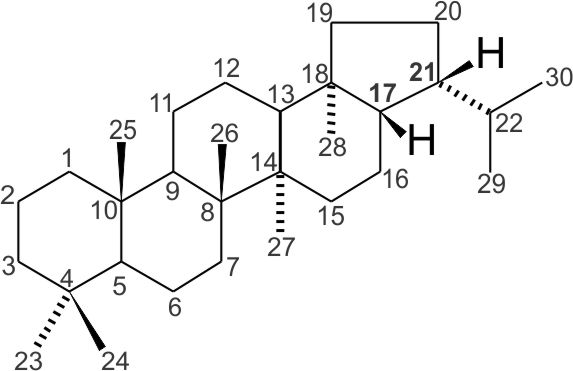
For example, the hydrocarbon hopane, a compound derived from functionalized bacterial hopanoids, has a biological configuration with hydrogens bonded to carbon atoms at positions 17 and 21 in beta (β) orientation (above the plane of the screen or paper) and are shown as bold wedges: 17β(H),21β(H)-hopane or in simplified notation 17β(H),21β-hopane. In the jargon of organic geochemistry this is a ββ-hopane.
|
Hopane |
17β(H),21β-hopane. The carbon atom number on the hopane skeleton is indicated. This numbering follows strict rules guides by the International Union of Pure and Applied Chemistry (IUPAC). |
|
C31-C35 hopane
|
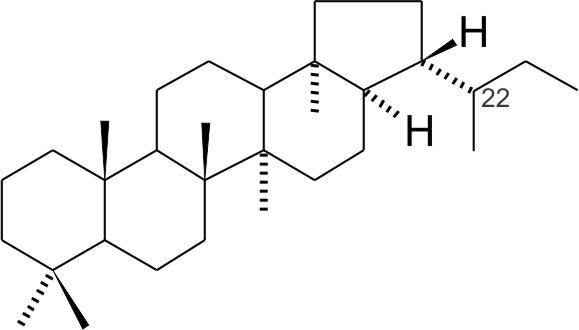
Upon mild burial and thermal stress the stereochemical configuration of such a hopane evolves to 17β(H),21α-hopane (βα-hopane) and with further burial to 17α(H),21β-hopane (αβ-hopane). Upon reaching the oil window all hopanes observed in sediments are in αβ configuration.
For hopanoid hydrocarbons with and an extended side chain, C22 is chiral and stereoisomerism around C22 occurs. The biological configuration is 22R and with increasing burial the 22S configuration appears until the S and R diastereomers are at equilibrium, usually at or just past the the onset of oil production. The ratio of 22S/(22S+22R) for homohopane (C31 hopane) is commonly used to characterize the level of thermal maturity of sediments that were subjected to low thermal stress. |
|
Further Reading
|
|